The History of eSTAR
The Food and Drug Administration Safety and Innovation Act (FDASIA) of 2012 included provisions requiring the FDA to improve the electronic submission process for 510(k) filings. The FDA started working on this new process in September of 2018, and the Center for Biologics Evaluation and Research (CBER) piloted an initial program called the Electronic Submission Template and Resource Pilot Program, which eventually evolved into eSTAR. The core of eSTAR is a PDF template designed to guide users through the 510(k) submission process. The goal here is efficiency, both for the FDA and for submitting organizations.
What eSTAR Requires
Until October 1, 2023, eSTAR will remain a voluntary process that is free to use for medical device applicants wishing to submit either a 510(k) or a De Novo to the CDRH, though it can’t be used for many other types of submission types yet. One key note is that standard submission fees for De Novo and 510(k) do still apply.
One thing that is not required for eSTAR submissions is Refuse to Accept (RTA) reviews. These preliminary reviews of submissions aren’t necessary for eSTAR submissions because the eSTAR template in some way works of this review checklist and is meant to aid submitting manufacturers know if their submission is complete. Incomplete eSTAR submissions simply won’t be reviewed.
Submission Guidelines
“Providing Regulatory Submissions for Medical Devices in Electronic Format - Submissions Under Section 745A(b) of the Federal Food, Drug, and Cosmetic Act” contains essential guidance regarding the development of templates for electronic submissions, which led to the creation of eSTAR templates and could be used for creating proprietary templates. “Electronic Submission Template for Medical Device 510(k) Submissions” contains final FDA guidelines for electronic submissions, including exemptions for situations where users may not need to use eSTAR. Consulting these documents is an essential first step in determining whether and how users need to submit via eSTAR.
Users can currently send in eSTAR submissions via two methods: Online via the Customer Collaboration Portal, or by mailing a digital copy of the eSTAR PDF on a USB drive, CD, or DVD. For mail-in submissions, users should download the eSTAR PDF template for either an In Vitro or non-In Vitro device and fill it out according to the directions. Once the template is complete, users can save a copy to send in by mail instead of submitting online. It is not necessary for eSTAR submission to comply with the FDA’s eCopy Guidance document, but any additional files beyond the eSTAR PDF itself will need to comply with these guidelines. Users also don’t need to include an Indications for Use page, a Premarket Review Submission Cover Sheet, or a Declaration of Conformity, since these documents are built into the eSTAR PDF. It is usually a good idea to merge all files into a single PDF document for mail-in submissions.
Note: Users who are submitting a 510(k) product change will need to include a justification for not including original information in sections that did not change, since the eSTAR template will still automatically require this information.
Using the eSTAR Template
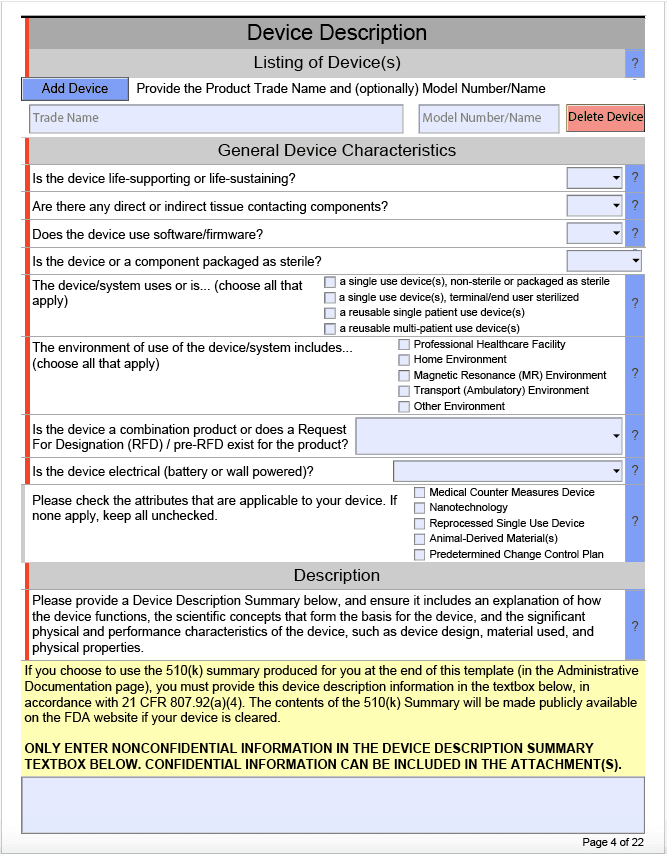
The above image is from the Device Description section. It is designed to prompt users for all required information by turning sections red if they need to be filled out, turning them green when they have been filled out, and turning them gray if they are optional. These forms also use cascading drop-down menus, meaning that as you answer some questions, others may automatically appear based on your responses. One key note here: Most submissions will require the inclusion of attachments. It’s a good idea to break these attachments up into small individual sections, since the form will not be able to re-use submitted files in different sections.
Tracking your Submission
The FDA’s Customer Collaboration Portal (CCP) will allow your Official Correspondent to track your submission’s progress. It will be necessary to create an account for this portal for first-time users.
Potential Challenges
Despite the improvements that eSTAR brings to the 510(k) submission process, there are organizational and assembly challenges associated with overall submission process that needs to be addressed. It is still early to look at FDA data that indicates 71% of submissions have led to additional information requests, 34% resulted in a refusal to accept, and 7% ended up on technical hold. This leads to a number of problems for businesses:
Submission delays. Especially with current regulations and procedures being in flux, businesses are constantly working to catch up and make changes to existing submissions.
High training costs. Managing the eSTAR system, other FDA regulations, and the submission tracking process all require a good deal of hands-on learning before employees can handle them without errors.
High overhead. Manufacturers will still need to manage their unique templates, handle countless attachments, and communicate with multiple stakeholders in order to comply with eSTAR regulations.
What Causes These Challenges?
The eSTAR form is quite cumbersome. While one would expect that the field-dependent structure of the form would make submissions much more straightforward in theory, this isn’t always the case in practice. Some fields fail to properly generate or populate on some devices or Acrobat readers. The PDF will also automatically blank out fields that are filled out of order or with incorrect information. The PDF is also not very effective at checking the quality of the information entered in each field. A red box is a clear sign that a field hasn’t been filled out, but it’s possible to enter incorrect information and get a green box from the PDF–leading to requests for more information and another round of back-and-forth.
The Acrobat eSTAR form is an offline document that doesn’t allow for collaboration. This means that teams have to either have a single person entering information on the eSTAR PDF, or carefully save and hand off the Acrobat form from one department to department. This leads to issues with embedding files, duplicating content efforts, and losing master copies and historical context. With up to seven different teams working on the average eSTAR submission, manufacturers often need to create an in-house template to collect and organize information before inputting it into the PDF.
Rapidly changing Medical device regulations are taking their toll on eSTAR maintenance burden for Regulatory affairs teams. Since 2018 when this initiative began, there have been several changes to the eSTAR framework roughly once every 2 months. This means that regulatory affairs teams are constantly forced to review their submissions and make changes to accommodate new regulations, as well as duplicating all the effort they put into internal documents and templates.
Attachments are confusing with eSTAR. Engineering drawings need to be compiled in a separate document, while other content needs to be included in the main document, and these requirements tend to change about as frequently as others adding the training burden for regulatory affairs teams.
Best Practices
So, with all of these issues, how can regulatory affairs teams keep up with eSTAR requirements?
Templates. One first step should be to create your own copies of the eSTAR templates for internal use. This will make it much easier to input the required information collaboratively.
File storage. Secure, cloud-based storage is essential for managing eSTAR submissions. The number, size, diversity, and potential sensitivity of these files mean that keeping them all in one place with controlled access is essential.
Planning. Create clear checklists with timelines to assign specific parts of the eSTAR process to the relevant departments, make sure the work gets done, and keep track of deadlines. The eSTAR process generally takes several days to complete as well as to be approved, so it’s essential to build in extra time.
Training and Standard Operating Procedures. Companies should plan on at least 2 weeks of training time for each regulatory change in eSTAR’s framework and requirements. Since these changes happen regularly, it’s wise to invest heavily in in-house training staff to handle this workload, and in-house documentation teams to create and update SOP documents.
About Essenvia
Essenvia is the only regulatory platform ready to streamline eSTAR submissions and walk you through each step in the eSTAR process. Regulatory Affairs teams are already using Essenvia to:
Save time and quickly adapt to eSTAR (including the new versions released by the FDA)
Reduce stress by co-authoring eSTAR submissions directly inside of the submission file, leading to fewer version mismatch and file fragmentation headaches
Ensure a 100% acceptance rate by the FDA
Watch this 3-minute video to understand how Essenvia can help you adapt faster to eSTAR or Schedule a demo now to find out how Essenvia can help you with your regulatory submissions.





